We design smart materials that organize themselves
The next generation of nanoscale devices need new nanomaterials
Our limited fossil fuel supplies and environmental concerns motivate the international quest for improved sustainable energy sources. Key energy technologies, including photovoltaics, batteries, pseudocapacitors, fuel cells, and solar fuels all rely upon electrochemistry with reactions occurring at controlled interfaces and multiple species migrating along continuous pathways. Here devices with sufficient power for practical devices require fast reaction rates and thus rely upon nanostructured materials having ample active interfacial area. The controlled formation of nanoscale functional materials is thus critical to advance numerous platforms for alternative energy.
 Smart materials ‘know’ how to self-assemble
Smart materials ‘know’ how to self-assemble
Block copolymers are a fascinating class of molecules that can self-assemble into a diverse range of structures. They can be thought of as chain-like molecules called homopolymers, for example A and B, that have been connected together. Generally, the enthalpic cost for A-B contacts drives phase separation; however, since the blocks are tethered together, they can only phase separate on the length scale of the chain, usually 1-100 nm. Researchers have brought this structure control to functional materials by including inorganic components. Numerous materials and morphologies have been made starting since the 1990s, so what challenges remain?
Making the right stuff
The nanomaterials revolution in the battery research community taught us a lot about architecture-performance relationships. Namely, length scale matters, a lot – a material that seemed useless yesterday can become tomorrow’s high performer, but only if the right nanoscale architecture is used. But what is the “right architecture?” All electrochemical devices convolve multiple concurrent processes so answering this question requires consideration of multiple parameter dimensions. A guess-and-check approach is valid, but it turns out to be very difficult to fabricate tunable series of nanomaterials. A more efficient approach is to make the right series of materials with specific constants that explore nanoscale phenomena along isolated dimensions. This approach allows one to hone in on new architecture-performance relationships for isolated processes such as charge insertion or charge transport. Thus our ability to learn more and eventually produce the right architectures comes down to a materials chemistry challenge: how can we make it?
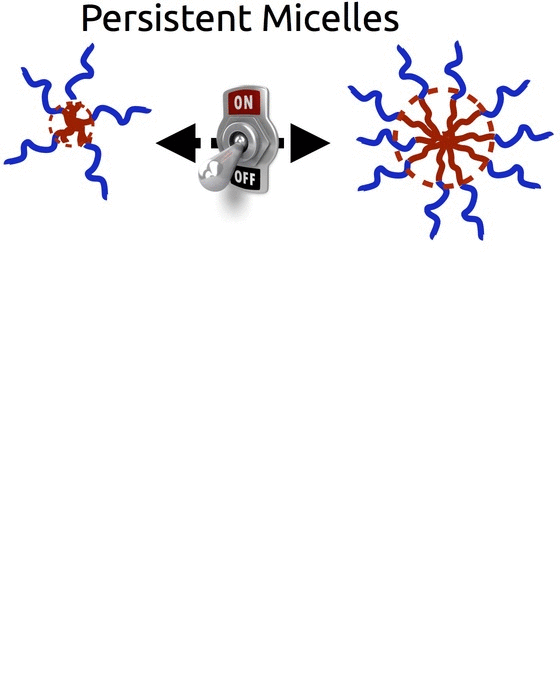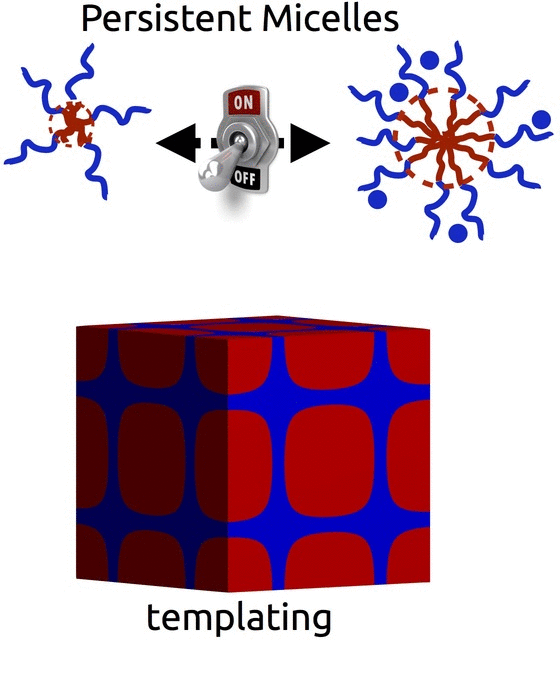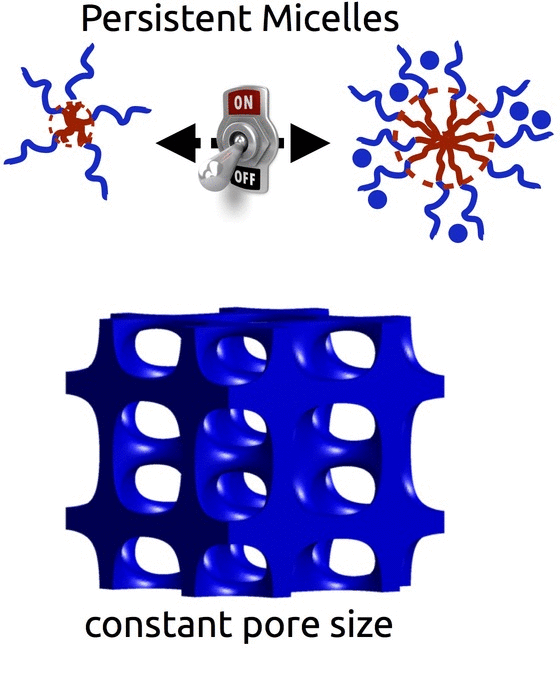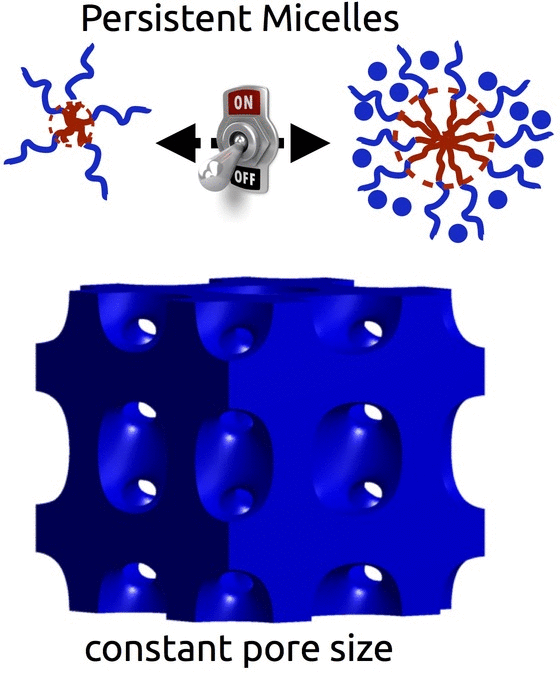
Persistent Micelle Templates (Patented)
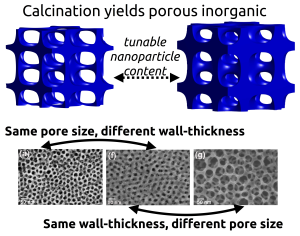
The self-assembly of block polymers is a powerful tool to realize functional materials with nanoscale features. The resulting porous materials all have a morphology with 2 characteristic dimensions : the material dimension and the pore dimension. However, in most synthesis approaches these two characteristics are coupled and there is often limited ability to vary either. With either of the two formation pathways (solution phase vs bulk phase), the use of equilibrating conditions is fundamentally incompatible with decoupled control – i.e. any change to the material chemistry also results in changes to the pore dimensions. Breaking free of this constraint requires the use of non-equilibrium conditions via e.g. kinetic entrapment.
We invented Persistent Micelle Templates to enable the next generation of energy devices. Here we use block copolymer micelles to template material precursors. You can add as much or as little material as desired without the micelles responding to the changing thermodynamic conditions. This control allows us to realize systematic series of materials where e.g. the pore size and morphology are held constant while the material wall thickness is monotonically varied with remarkable granularity, as recently reviewed. Such tunable isomorphic architectures uniquely enable the investigation of electrochemical devices. Our recent studies have examined the nature of intercalation pseudocapacitance and our ongoing work is examining new active materials for batteries.